CIRSE 2014: Hybrid treatment with angiographic catheter in massive pulmonary embolism: mechanical fragmentation and fibrinolysis
Stefano PIERI, Paolo AGRESTI
Purpose
Massive pulmonary embolism is a severe clinical condition, that requires prompt therapeutic intervention.
Medical and surgical interventions are ineffective.
 We report our experience with a hybrid treatment, involving systematic fragmentation of the embolus with an angiographic catheter associated with fibrinolytic therapy over the following days.
We report our experience with a hybrid treatment, involving systematic fragmentation of the embolus with an angiographic catheter associated with fibrinolytic therapy over the following days.
Methods and materials
Between 1999 and 2012, 628 patients were referred to our service for treatment of pulmonary embolism. Of these, 235 had a massive pulmonary embolism, with haemodynamically instability.
The most common risk factors included a recent history of surgery (70 pts – 29,7%), prolonged immobilization (47 pts. – 20%), hypercoagulation syndrome (56 pts. – 23,8%), a combination of several factors (62 pts. – 26,3%).
Diagnosis was made with computed tomography.
Inclusion criteria for the treatment were hypotension (<90 mmHg), angiographic confirmation of massive pulmonary embolism (involvement of the central pulmonary arteries), flow diversion towards the controlateral side, mean pulmonary pressure > 35 mmHg
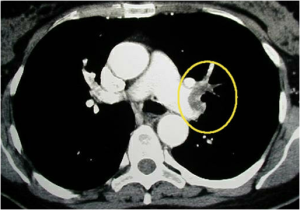
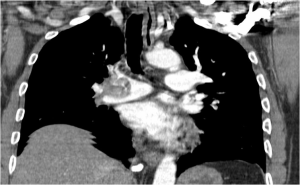
Fig . 1 – Computed tomography of the chest with pulmonary embolism on the left
The hybrid treatment consists of a diagnostic phase and an interventional one.
Pulmonary angiography was preferentially performed with a transbrachial approach.
The introducer sheath was 7 Fr, 45-65 cm – hydrophilic guide wire 180 cm, 0,035”, 3 cm floppy tip, pig-tail 6Fr, 100-120 cm, 145°.
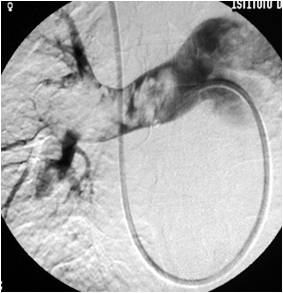
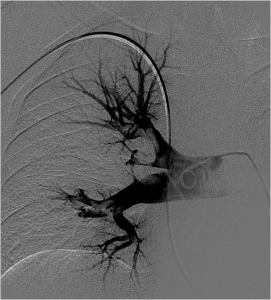
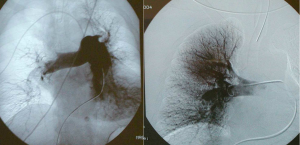
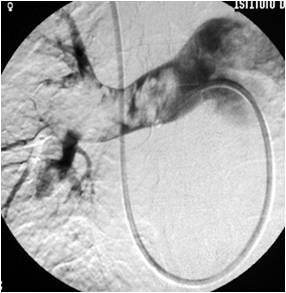
FIG. 2 – Selective catheterization of the right pulmonary artery with massive pulmonary embolism
The pig-tail catheter was used to administer the contrast medium (40ml at 20 ml/sec in the pulmonary arteries, 30 ml at 15 ml/sec in the lobar arteries).
To measure the mean pulmonary artery pressure.
To guide the interventional procedure.
FIG. 3 – Selective catheterization of the left pulmonary artery from transfemoral approach
The interventional phase involved administration of urokinase (300.000 UI) from the angiographic catheter.
The caudal part of the guide wire was inserted into the catheter, to about 1-2 cm over the first angle, on order to provide the necessary stiffness to the entire device
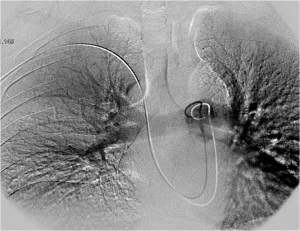
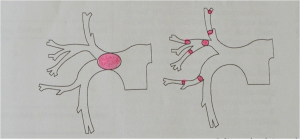
Under direct fluoroscopy control, the angiographic catheter was rotated clockwise inside the pulmonary arteries (5-10 rotations); at the same time advanced and withdrawn in a craniocaudal direction.
FIG. 4 – Rotational movement of the angiographic catheter.
The rotating motion was associated with the friction one.
Each treatment consisted of 10 peripheral rotations; mechanical fragmentation was repeated with further cycle of ten rotations at a time, if pulmonary artery pressure failed to drop below to 30 mmHg after the first passage.
FIG. 5 – TC of the lungs with massive pulmonary embolism in the right pulmonary artery.
Immediate outcome was considered good when mean pulmonary artery pressure fell to 25 mmHg, after the first passage, moderate if fell to 30 mmHg, poor if fell to 35 mmHg.
In the following three days, 50.000 UI/h urokinase were administred directly from the angiographic catheter.
Pulmonary angiography and hemodynamic follow-up were assessed ever 24 h.
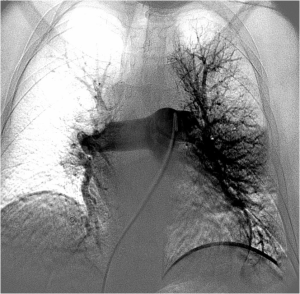
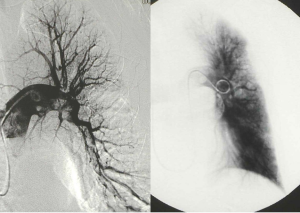
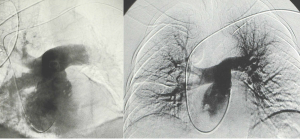
FIG. 6 – Selective angiography after the first passage of the rotating motion: the big embolus was fragmented into three prts.
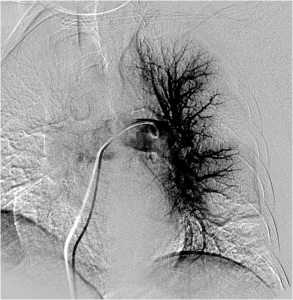
FIG. 7 – Global pulmonary angiography after three days of therapy.
Results
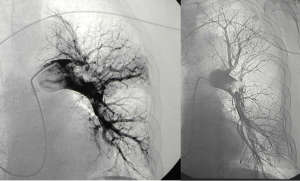
Fig 8 – I group before and after the treatment
After fragmentation and administration of fibrinolytic agent we found 4 types of haemodynamic behaviour: in 114 pts (48,6%) the mean pulmonary pressure
fell rapidly below 30 mmHg.
Fig 9 – II group before and after the treatment
in 57 pts (24,3 ) two passes were required to achieve the same result ( mean pulmonary pressure below 30 mmHg).
Fig 10 – III group before and after the treatment
In 37 pts (15,8% more than three passages were required to reach 30 mmHg.
Fig 11 – IV group before and after the treatment
In the remaining patients at no time did the meanspulmonary artery fall below 35 mmHg.
The only four deads occurred in this last group.
Discussion
The different results of the fragmentation can be explained with the different beahaviour of the emboli that we encountered:
with phisiologic retraction the clot loses more than 90% of its plasminogen content and becomes increasingly resistant to thrombolytic treatment.
The rapid drop in pressure values during or at the end of the mechanical fragmentation can predict the outcome of the therapy.
Continuous infusion of the fibrinolytic agent after the fragmenatation is performed because it is able to further improve haemodynamic condition by exploiting the greater surface area exposed to the fibrinolytic agent, after the mechanical fragmentation.
Conclusion
In patients with massive pulmonary embolism, mechanical fragmentation with a conventional pig-tail catheter and the addition of intrapulmonary fibronolysis proved capable of rapidly improving severe cardiopulmonary insufficience.
This simply and minimally invasive therapy is capable to produce surprising results: it enables the patient to return to normal or near normal mean pulmonary artery pressure values extrememely rapidly.